Efficient
Effective
Compliant
Sustainable
Value-Driven Personalized Solutions
Efficient
Reducing redundant paperwork with Integrated Risk Based C&Q.
Effective
Perpetuating efficiencies throughout system life cycle, quality by design.
Compliant
With GMP regulations, data integrity and data security. VEQTOR provides in-house training program to all our employees.
Sustainable
Service agreements with two dozen leading pharmaceutical companies.
Value-Driven Personalized Solutions
Welcome to VEQTOR Technical Services
For The Life Science Industry

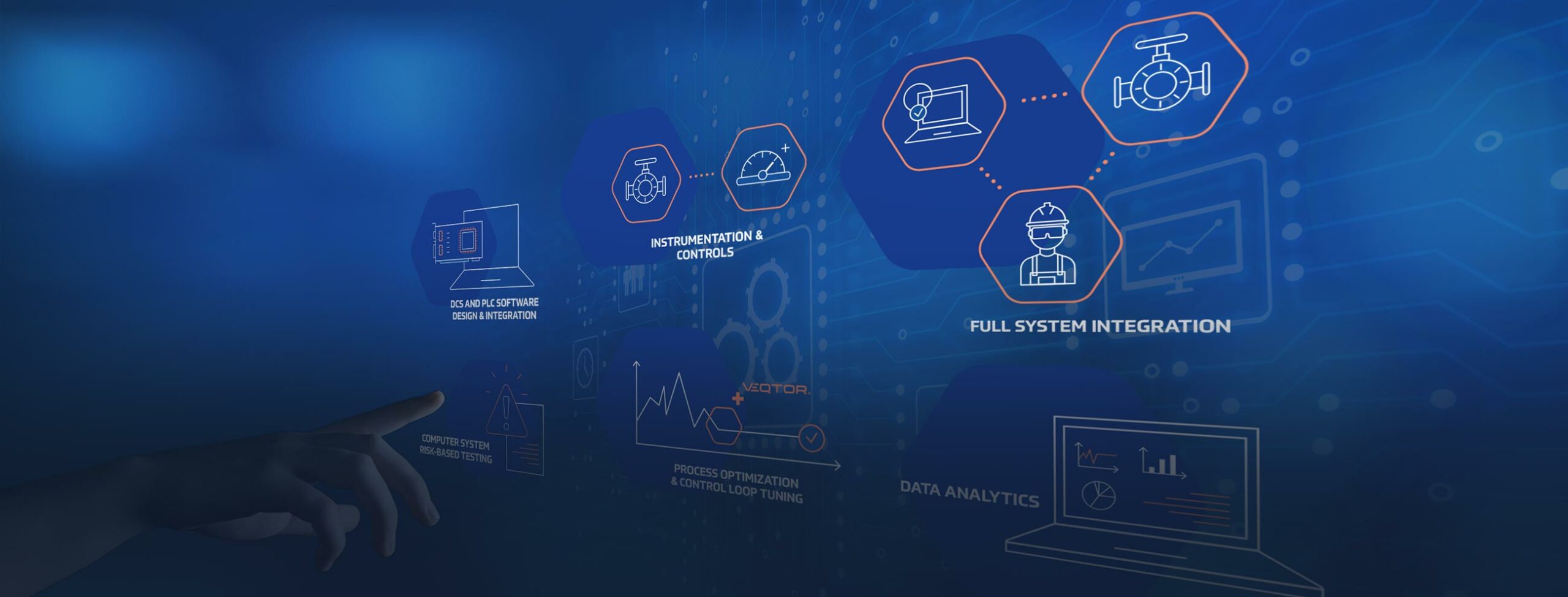
ABOUT US
As a technical service provider in the Life Sciences Industry we are committed to reducing time, cost, waste, and redundancy without impact to quality and compliance.

BUILDING STRATEGIC
PARTNERSHIPS
Our partnerships are strategically chosen to ensure we meet our clients current and future needs.
INNOVATORS IN LIFE
SCIENCE PROJECT
MANAGEMENT
VEQTOR serves the unmet needs and commercialization challenges of the Life Sciences Industry to develop life-saving therapies. Our services ensure manufacturing is safe and designed as intended.


WHAT WE DO?
We deliver solutions to facilitate both present and future growth for our clients within their existing system.

ADVANCE YOUR CAREER
WITH US